GoldFinch for Pharmaceuticals
GoldFinch ERP leans on comprehensive, industry-specific functionality to help pharmaceutical businesses adapt to marketplace changes and grow without operational disruption.
Trusted by Pharmaceutical Leaders
Batch Validation and Quality Control Management —Designed for the Pharmaceutical industry
Production and distribution of over-the-counter and prescription drugs require exceptional control over complex processes and regulations.
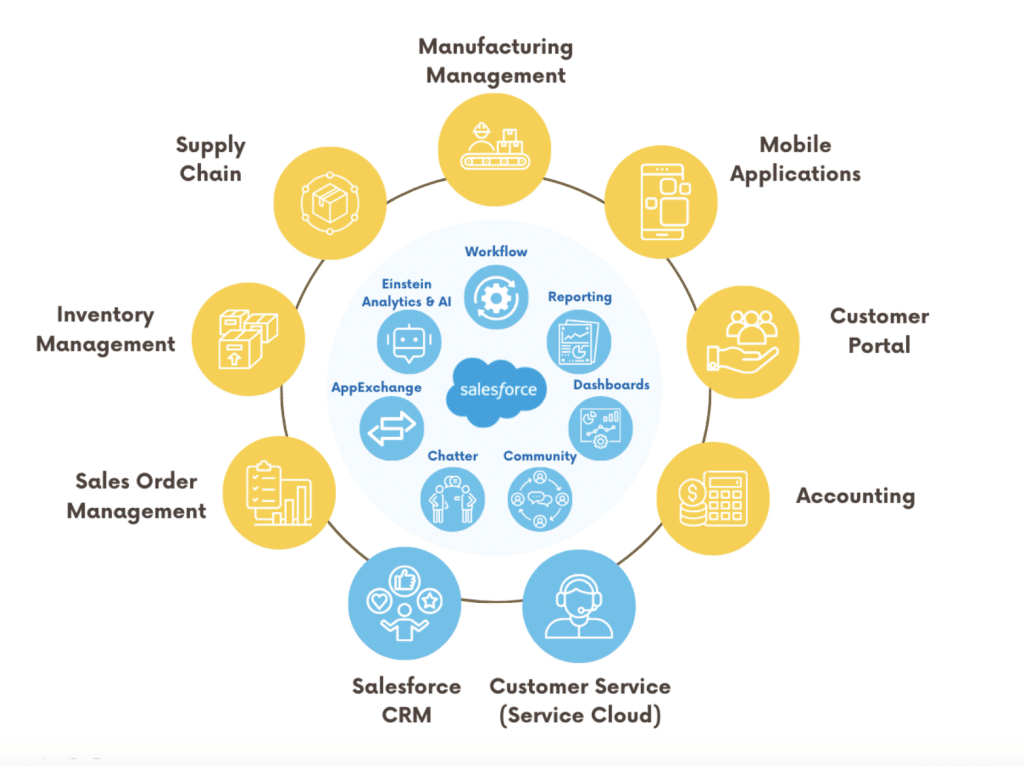
Built natively on Salesforce, GoldFinch ERP is uniquely tailored to address the intricate requirements of pharmaceutical companies involved in distribution and manufacturing.
With our deep understanding of the pharmaceutical industry, we offer specialized features and functions to:
- Ensure compliance with strict regulations
- Enable meticulous batch tracking
- Make robust quality control simple

Download Your Free Brochure
Discover how GoldFinch ERP & Accounting simplifies operations and strengthens compliance in Health & Life Sciences. Click below to register for your free brochure.
Do efficient business, your way, worry-free.

Regulatory
Compliance
In the pharmaceutical industry, compliance matters from efficient and secure document storage, to recall management and even change control. GoldFinch ERP gives you the power to ensure compliance on every front, all the time.

Meticulous
Batch Tracking
GoldFinch ERP facilitates batch and lot traceability, linking certain batches to their expiration dates, and providing automated alerts and notifications so you’re always informed about impending ingredient expirations.

Streamlined
Recipe Management
Whether you’re worried about altering manufacturing processes, recalibrating dosages, or testing new formulations, you can ensure they met the heath and safety standards across any region you operate in.
Lean on specialized ERP features purpose-built for pharmaceuticals.
- Track multi-level formulations
- R&D functionality and Quality Control Management
- Formula version management system
- Product Recall process
- Work Center Management & MRP
- Deep lot tracking, tracing and serialization to the batch component level
- Effective batch sizing and scaling percent or quantity
- Wholesaler deduction management
- Ability to meet compliance requirements: FDA and cGMP
- Employee certification control
- CFR/21 Compliance for Electronic Batch Records
- ALCOA+ Principles natively supported
- Management of Salesforce security and audit trails





