GoldFinch for Medical Device Manufacturing
GoldFinch helps growing manufacturers manage the development of medical devices —with complete precision to support regulatory compliance.
Trusted to support niche industry requirements by medical device manufacturers.
Streamline processes with precision —from raw materials to finished products.
With GoldFinch ERP, you get the control, flexibility, and scalability you need to succeed in an ever-changing regulatory environment.
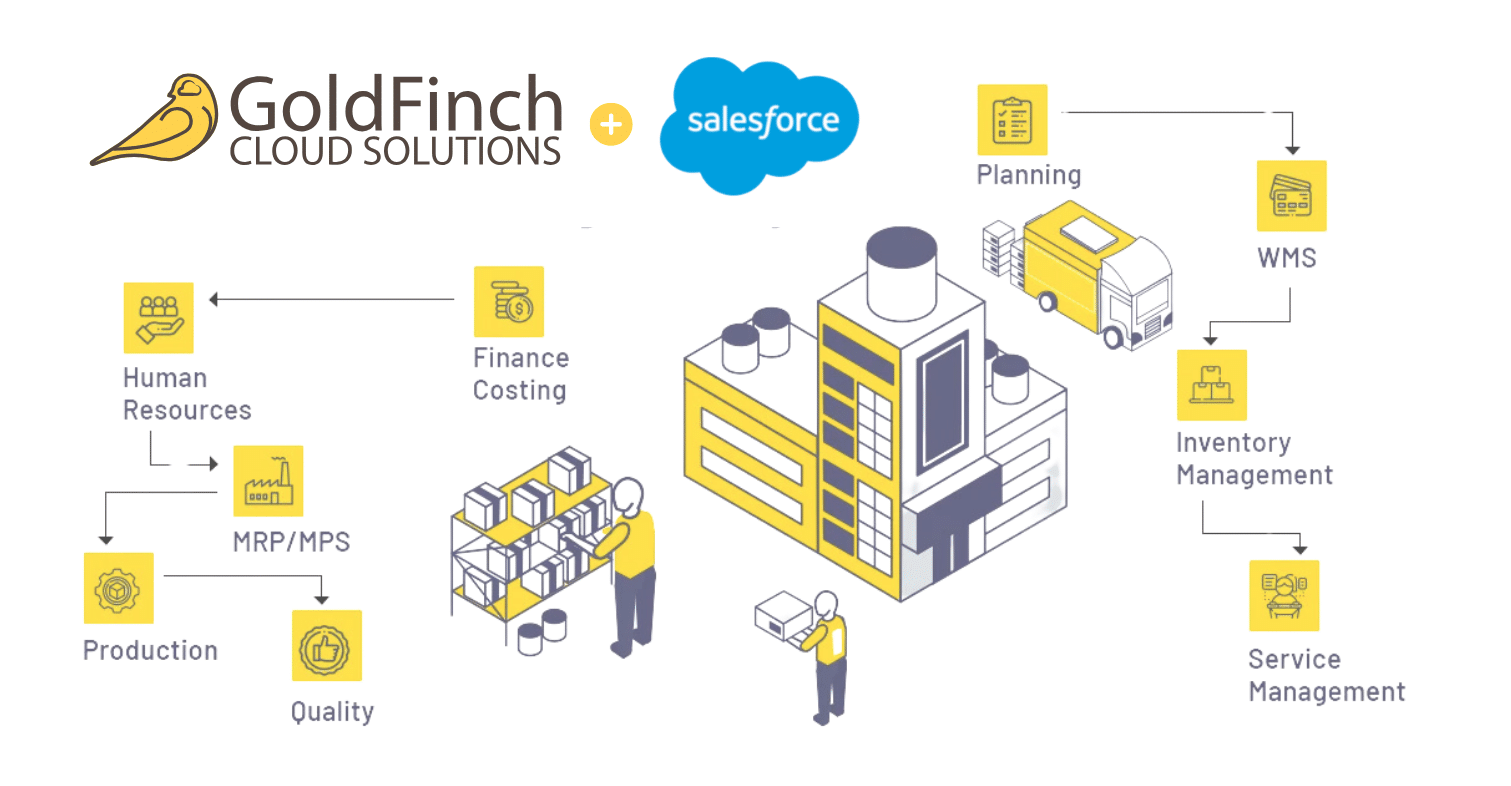
Our comprehensive system addresses the complexities of medical device manufacturing with the native power of Salesforce — for a seamless integration that ensures efficient operations, refined inventory management, and heightened productivity.

Download Your Free Brochure
Discover how GoldFinch ERP & Accounting simplifies operations and strengthens compliance in Health & Life Sciences. Click below to register for your free brochure.
Experience accurate, efficient, and streamlined procedures.

Get Total Operational Visibility
Centralize your data and let your teams access it in real-time, from finance to supply chain and human resources. With GoldFinch’s ERP solution, you can eliminate inefficiencies, manage costs, align teams, and streamline processes from one single source of truth.

Maintain Compliance
GoldFinch ERP offers functionality like batch tracking and extensive Salesforce audit trails to ensure you comply with regulations and can handle circumstances like product recall management with ease.

Ensure Product Quality
For medical device manufacturers, safety and dependability are non-negotiable. With GoldFinch, bring accuracy and precision to your processes with real-time data, production QA testing, and automated inventory management.
Lean on specialized ERP features built just for medical device manufacturers.
- Meet FDA regulatory compliance with lot and serial number traceability
- Comply with medical device barcoding regulations using native Salesforce Mobile code
- Use barcoding to trace parts, components, and completed products for comprehensive inventory control
- Securely store compliance, design, and engineering documentation within Salesforce
- Enhanced product recall compliance
- Secure, centralized data storage with mobile accessibility
- Advanced accounting for better financial visibility
- Comprehensive Materials Requirement Planning (MRP) to suggest replenishment orders
- Real-time data for improved sales and supply forecasting
- Employee certification tracking
- Quality Assurance certification management
- Work Center Drag & Drop capacity scheduling
Explore more resources

Managing Medical Device Updates With ERP
Medical devices need to be updated from time to time. Such types of equipment are heavily regulated to ensure they meet safety standards. As such, manufacturers have to update these…

Developing Your Next Medical Device with ERP
Medical device manufacturing is among the US’s fastest-growing and most competitive industries. It makes it crucial for manufacturers to streamline their operations by leveraging intelligent systems and technology. Most medical…

ERP for Medical Device Manufacturers
Discreet manufacturers need systems to optimize and streamline their operations for quality products. These systems automate core operations, organize your data, optimize the supply chain, and ensure your efforts are…